
To get a true insight into nature of Maple one needs to explore the tiniest details of behaviour of all its 3500+ functions, in all Maple versions.
|
|
To get a true insight into nature of Maple one needs to explore the tiniest details of behaviour of all its 3500+ functions, in all Maple versions. |
|
On April 19th 1894 Ramsay heard Lord Rayleigh lecture to the Royal Society, when he pointed out that nitrogen isolated from the air had a density slightly higher than that of nitrogen prepared from chemical sources. A litre of pure nitrogen gas generated from a chemical reaction weighed 1.2505 g. On the other hand, a litre of nitrogen gas generated from air by removing oxygen, carbon dioxide, and water vapour weighed 1.2572 g (at the same temperature and pressure). Rayleigh thought that this might be due to the presence of a light impurity in the former (Rayleigh's paper is available online). But Ramsay thought that it might be due to the presence of a heavy impurity in the "atmospheric" nitrogen. He was an enthusiast for the Newland/Mendeleev Periodic Table and we have Mendeleev's signature in one of our books of visitors. He thought that there might be an unrecognized new element hiding in the air, and that there might be room for this in a new Group at the end of the Periodic Table. |
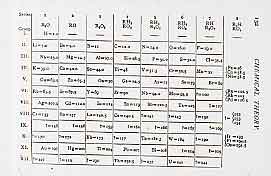 The Periodic Table before Ramsay's discovery.
The Periodic Table before Ramsay's discovery. |
When examinations had finished, Ramsay
set about attempting to isolate this "impurity".
Ramsay's Apparatus for Isolating Argon |
A
picture of the Argon Apparatus taken from his notebooks.
Argon
Isolation |
|
Ramsay's paper on the discovery is also available online. (We are grateful to Prof Carmen Giunta for putting the above papers online). |

|
© 2003-2010 Cyber Tester Ltd.
All rights reserved. All logos and trademarks are property of their respective owners.